Pharmaceutical Oscillating Granulator Machine / Extruder 380V 220V
Product Details:
| Place of Origin: | China |
| Certification: | SGS CE ISO |
| Model Number: | YK |
Payment & Shipping Terms:
| Minimum Order Quantity: | 1units |
|---|---|
| Price: | USD1900-3890 per unit |
| Packaging Details: | plywood or shipping container |
| Delivery Time: | 25 working days |
| Payment Terms: | Western Union, MoneyGram, D/A, L/C, D/P, T/T |
| Supply Ability: | 1000units per year |
|
Detail Information |
|||
| Material: | SUS304 SUS316L Food Grade GMP | Standard: | GMP |
|---|---|---|---|
| Product: | Granules | Cutter: | Speed Viable |
| Color: | Stainless Steel | Voltage: | 380V 220V |
| Granule Size: | 0.2mm-3.5mm | ||
| Highlight: | Pharmaceutical Oscillating Granulator Machine,380V Oscillating Granulator Machine |
||
Product Description
Pharmaceutical Machine / Extruder/ shear granulator/ Oscillating Granulator for Food and Pharma
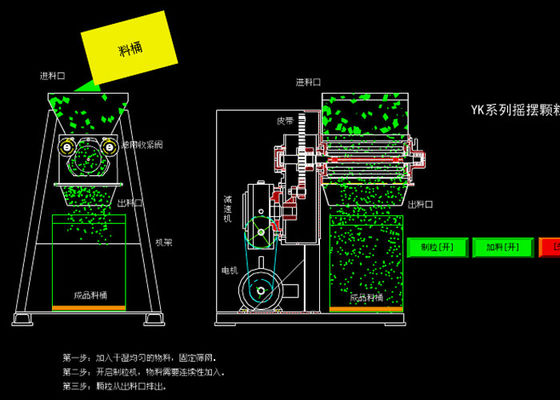
Oscillating granulator Working Principle
Swing granulator/ pelletizer can be mixed powder was made of particles, can also be crushed dry bulk material into the desired granules. For the pharmaceutical, chemical, food, research institutes, laboratories, hospitals and small health care products plant small batch production.
It can make well stirred raw materials become granules of required size of crush the blocked raw materials small granules.
Material to be dried
The machine is applicable for pharmaceutical,foodstuff,chemical,solid drink and so on lndustries.
![]()
Technical Parameter
| spec | YK160 | YK320 |
| capacity | 50-200 kg/h | 50-400 kg/h |
| power | 2.2kw | 7.5kw |
| speed | 65rpm | 65rpm |
| sway angle | 360o | 360o |
| diameter | Φ160mm | Φ320mm |
| weight | 450kg | 750kg |
| overall dimension | 1000×800×1300m | 1500×1200×1500m |
Prepare process 1.1 previously used in the production of raw materials for processing according to process requirements; 1.2 ready for raw materials used in production and a binder or wetting agent; 1.3 checking the quality of raw materials used in production meets the requirements; 1.4 inspection the shift clearance meets the requirements, all the utensils used in the production of container is clean. 1.5 ready already qualified verification of measuring instruments. 2. The mixing process raw materials into a mixer operating 2.1 container; 2.2 first start mixer, raw materials were dry-mixed; 2.3 mixing a predetermined amount of adhesive quality. Or according to process requirements corner binder while mixing granulation; 2.4 requirement is to be reached, stop the tilt release of soft material, then the soft material with splice car; granulation process 3. Operation 3.1 After mixing soft material, according to process requirements Shang Hao net. 3.3 granulated material mixing is complete, the remainder of the container; adding a small amount of soft material test, size meet the requirements before feeding; 3.2 granulated particles should always check the firmness of whether two long strips and tightness of the phenomenon clean; 3.4 Press "swing granulated clean standard operating procedures" for cleaning; 3.5 Fill in production, cleaning, clearance record. 4. Evaluation of the results of operations 4.1 Checking the appearance of wet granulation, shall comply with the requirements; 4.2 wet particle size, should comply with the requirements; process control process 5. Operation 5.1 Operating composite least two, review the feeding of raw materials or adhesives wetting agent names, numbers are consistent with the production orders; 5.2 mixing must be uniform production process; the process 6. Handling Precautions: 6.1 each feeding, only joined by container volume raw materials 2/3. 6.2 checking nets damaged, do not hand touch. We found a large piece of soft material or particles fall, the proof nets have been broken, the need to stop immediately replaced. When the machine in a foreign body, must take the hand, must be stopped excluded.
Item 7. The procedure used equipment, appliances goods raw materials, adhesives or wetting agents
Title swing granulation SOPs coding: PO-005-00 Page: 1/2 formulate approval date of enactment of the date of approval issued by the department of audit date GMP Office issued effective date number three distribution unit production unit granules / tablets / capsules